Well, wonders will never cease. Not only is water already one of the strangest,and most interesting fluids/substances around, but clever scientists from the University of Tokyo just added another layer of WTFness.
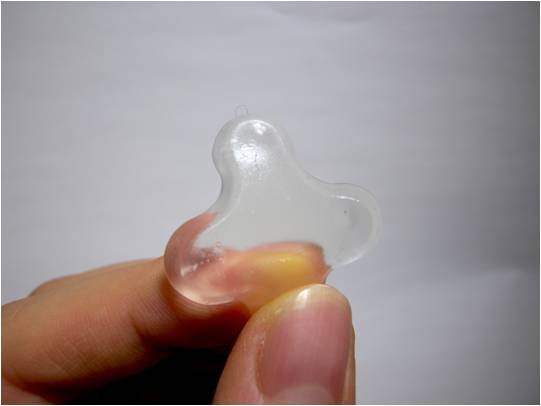
In essence, they have developed a sort of non-fluid, yet still transparent and flexible, water. A hydrogel. That’s flexible. And transparent. (That was worth repeating). I’ve seen a reference on the web to ‘elastic water’ but thought that was pushing it a bit far.
Published in the Jan 21 edition of Nature, the paper outlines how the researchers were able to create a high-water-content hydrogel using only water, a bit of clay, and a pinch of organic components (details below).
‘But why’, I hear you ask. ‘We already have other plastic materials - they’re called, you know, ‘plastic”, I hear you say. And that’s the problem - they’re all based on petroleum, which is based on oil, which is an increasingly unsustainable thing on which to be based. You know, what with the inevitability of world oil supplies beginning to decrease and stuff.
So scientists feel it’s reasonable to start exploring other means of constructing plastic materials. Plastic in the true sense of the word: flexible and mouldable. Hydrogels - flexible water-based gels - seem an obvious thing to start looking into (and of course we won’t get into the water debate here).
The recipe for this hydrogel goes something like this: take some water. Add about 2-3% by mass of clay. Mix, and add 0.4% by mass of certain organic components*. Shake well, at least metaphorically, for 3 minutes or a bit longer.
And voila! The final product is a transparent hydrogel with some very interesting properties. It’s able to stick together, which means it can easily be built into structures etc. It also keeps its shape, so any structures it’s used for can be free-standing - all due due to its ‘outstanding mechanical strength’.
It’s able to self-heal when damaged, and preserves biologically active proteins for catalysis (great for setting up reactions involving enzymes). In fact, it has some interesting applications for building reaction sequences using blocks containing different enzymatic activities.
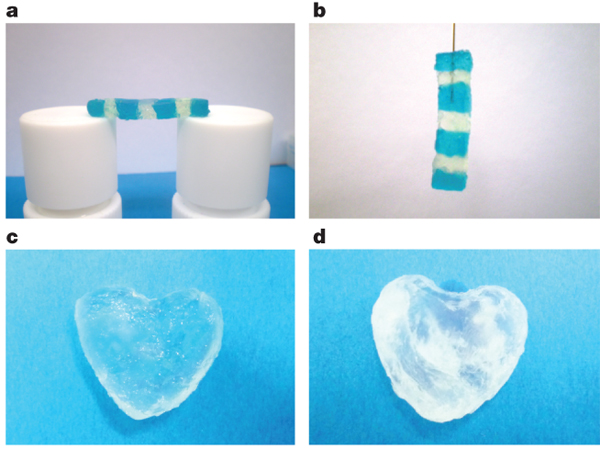
Most hydrogels have poor transparency, are brittle and can’t self-heal. In addition, making them is an involved process of multiple iterations of heating and cooling, agitation using sound, and in situ polymerisation or crosslinking reactions. Our little hydrogel, however, is the polar opposite. It’s transparent, flexible, and great for building structures with. It’s easy to make - All one requires is water, the three ingredients, and mixing at room temperature for a few minutes (as few as 3). In addition, it’s able to persist in briny or pH-positive/negative (acid or alkaline, folks) conditions, and can, with the addition of a couple more compounds, even be made using salt water itself.
I mean c’mon - it’s even environmentally friendly! I’d take this hydrogel home to meet the parents, as it were.
In short, this hydrogel is going where no hydrogel has gone before, and kudos goes to Wang et al - great work, guys.
Reference:
Wang Q, Mynar JL, Yoshida M, Lee E, Lee M, Okuro K, Kinbara K, & Aida T (2010). High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature, 463 (7279), 339-43 PMID: 20090750
*In their words: “CNSs, a dendritic macromolecule (Gn-binder; n, generation number) and sodium polyacrylate (ASAP)”